AG Membranproteine
Current Research Projects
funded by Land Sachsen Anhalt, DFG: SFB 610 and NovoNordisk©
Overview
Membrane proteins occupy key positions in the communication between the extracellular and the intracellular space and attract pharmaceutical interest as therapeutic targets. Structural information for a rational drug design is scarce despite of growing efforts in this area. In most cases the low abundance of the proteins in natural sources is the limiting factor. To get mg amounts of protein for structural investigations we use over expression in E. coli as inclusion bodies and in-vitro translation systems. Functional protein can then be further characterized by biophysical methods. A stabilization of membrane proteins by reconstitution into liposomes or Nanodiscs™ is also pursued. We are currently working mainly on three different protein families, G-protein-coupled receptors (GPCRs) of class B, GPCRs of class A and carriers for organic solutes.
Class B GPCRs
Two members of this class are involved in wide spread metabolic diseases: the Parathyroid hormone receptor (PTHR1) and the Glucagon-like-peptide-1 receptor (GLP1-R) which play a role in osteoporosis and diabetes, respectively. A special feature of these seven transmembrane receptors is a large N-terminal domain with few highly conserved amino acid residues, including six cysteins which form three disulfide bridges essential for ligand binding (Fig. 1). This domain is a valuable modelsystem to test different refolding procedures. Upon ligand activation the signal is transmitted via the transmembrane part to the intracellular C-terminus where binding of downstream effectors is induced. Analogous to the production of the receptors, their specific ligands, the intracellular binder arrestin and variants of both are also expressed in E. coli as tools to develop functional assays, synthesize isotopically labelled compounds or affinity purify the receptors.
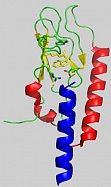
Fig.1: nGLP-1 receptor (Runge et al. 2008)
Class A GPCRs
Beside the overall membrane topology there is no striking homology of class A and class B GPCRs. The family of the Y receptors also bind peptides as ligands and are involved in pathologic processes. Y1 and Y5 receptors play a role in nutrition, Y2 is probably linked to the genesis of epilepsy.
Epithelial membrane transporters
The intestinal H+/peptide symporters PEPT1 and PEPT2 are of both physiological and pharmaceutical relevance. In addition to the transport of products from the breakdown of proteins they are carriers for a whole bunch of drugs, e. g. b-lactam antibiotics. Secondary structure prediction results in 12 putative transmembrane helices with the N-terminus located on the extracellular and the C-terminus on the intracellular side. A related carrier, the proton-dependent amino acid symporter PAT1, takes small neutral amino acids, prolin and GABA like compounds through the membrane and is associated to the inherited disease iminoglycinurie. The amino acid sequence is known and a topology with eleven membrane spanning helices is predicted.
Methods
The repertory of methods reaches from molecular biology to create appropriate expression constructs over standard biochemical work to a variety of biophysical techniques. Key competences are fermentation of up to ten litre cultures, in vitro-translation systems and development of refolding protocols and reconstitution methods. The spectrum is complemented by an environment with excellent facilities for structural studies.
References
Grauschopf, U.; Lilie, H.; Honold, K.; Wozny, M.; Reusch, D.; Esswein, A.; Schäfer, W., Rücknagel, P.; Rudolph, R. (2000) Biochemistry 39, 8879-8887 The N-Terminal Fragment of Human Parathyroid Hormone Receptor 1 Constitutes a Hormone Binding Domain and Reveals a Distinct Disulfide Pattern
Bazarsuren, A., Grauschopf, U., Wozny, M., Reusch, D., Hoffmann, E., Schaefer, W., Panzner, S. & Rudolph, R. (2002) Biophys. Chem. 96, 305-318 In vitro Folding, Functional Characterization, and Disulfide Pattern of the Extracellular Domain of Human GLP-1 Receptor
Lopez De Maturana, R., Willshaw, A., Kuntzsch A., Rudolph, R., Donnelly, D. (2003) J Biol Chem 278, 10195-10200 The isolated N-terminal domain of the glucagon-like peptide-1 receptor binds exendin peptides with much higher affinity than GLP-1.
Klose, J., Fechner, K., Beyermann, M., Krause, E., Wendt, N., Bienert, M., Rudolph, R., Rothemund, S. (2005) Biochemistry 44, 1614-1623 Hexa-histidin tag position influences disulfide structure but not binding behavior of in-vitro folded N-terminal domain of rat corticotropinreleasing factor receptor type 2a.
Runge, S., Schimmer, S., Oschmann, J., Schiodt, C. B., Knudsen, S. M., Jeppesen, C. B., Madsen, K., Lau, J., Thogersen, H., Rudolph, R. (2007) Biochemistry 46, 5830-5840 Differential structural properties of GLP-1 and exendin-4 determine their relative affinity for GLP-1 receptor N-terminal extracellular domain.
Parthier, C., Kleinschmidt, M., Neumann, P., Rudolph, R., Manhart, S., Schlenzig, D., Fanghänel, J., Rahfeld, J. U., Demuth, H. U., Stubbs, M. T. (2007) Proc Natl Acad Sci USA 104, 13942-13947 Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor.
Runge, S., Thøgersen, H., Madsen, K., Lau, J., Rudolph, R. (2008) J Biol Chem 283, 11340-11347 Crystal structure of ligand-bound glucagon-like peptide-1 receptor N-terminal extracellular domain.
Bosse-Doenecke, E., Weininger, U., Gopalswamy, M., Balbach, J., Møller Knudsen, S., Rudolph, R. (2008) Protein Expr Purif 58, 114-121 High yield production of recombinant native and modified peptides exemplified by ligands for G-protein coupled receptors.




