Forschungsprojekte Abt. Technische Enzymologie
Research activities
The group is concerned with research problems relevant to the application
of enzymes in industry or other fields of practice.
The present research work is concentrated on
1. mechanisms of protein stabilization
2. phospholipase D and phospholipid transformations.
1. Mechanisms of protein stabilization
Despite its great importance for application, the mechanisms of protein stabilization toward denaturing influences have not yet been sufficiently understood. Previous studies of our group had led to a model which postulates one structural region on the surface of the protein molecule where unfolding of the native protein structure under denaturing conditions starts. Strengthening this region by immobilization or protein engineering can result in a significant increase in stability of the whole protein molecule. Labile structural regions (such as external loop regions) are also assumed to play a key role in irreversible enzyme inactivation processes such as misfolding, aggregation, or proteolytic degradation. In our group techniques combining limited proteolysis with SDS-PAGE with densitometric evaluation, HPLC, N-terminal protein sequencing, and mass spectrometry have been
established to determine
- primary proteolytic cleavage sites which characterize the accessibility of
local structural regions, - proteolytic constants,
- global unfolding constants.
Recently, these methods have been used in combination with site-directed mutagenesis and spectroscopic techniques to characterize local structural regions of ribonuclease A and neutral protease from {Bacillus stearothermophilus.
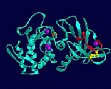
Model of the neutral protease from Bacillus stearothermophilus.The unfolding region is drawn in red colour. The disulfide bridge is drawn in yellow colour. The large spheres indicate the zinc ion (orange) in the active site and the four the calcium ions (purple) bound to the molecule.
2. Phospholipases and phospholipid transformations
Due to their manifold functions in nature, phospholipases are of great significance in biochemical basic research. Furthermore, they are important biocatalysts for the production of various phospholipids in laboratory as well as in industrial scale. Under the biotechnological aspect, the group is concerned with phospholipase D and phospholipase A2 and their biocatalytic potential. Phospholipase D is appropriate to modify the polar head group in phospholipids and phospholipid analogs, while phospholipase A2 is used for the production of lysophospholipids. Recently, phospholipase A2 has been considered for reacylation reactions to obtain phospholipids with defined fatty acid structure. At present, the production and characterization of the recombinant enzymes (phospholipase D from cabbage and phospholipase A2 from bee venom, expressed in E. coli) is studied. The results will provide the basis for improvement of stability and catalytic properties of these enzymes in organic media.
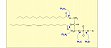
Figure 2
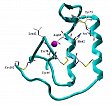
Figure 3
3. Ribonucleases as potential tumor therapeutics

Figure 4





