AB Enzymology - Peptidyl Prolyl cis/trans Isomerases
Because of the restricted rotation around the C-N bond, peptide bonds adopt two preferred conformations, the cis conformation and the trans conformation. As a specific feature of peptide bonds preceding proline (prolyl bonds), the similar steric situation in both isomers results in the coexistence of trans and cis isomers in nearly equal proportions in unstructured peptides.
Because of the high energy barrier for the rotation of the C-N bond the cis/trans isomerization of prolyl bonds is a slow reaction.
Peptidyl prolyl cis/trans isomerases (PPIases) are ubiquitous enzymes catalytically accelerating the interconversion of the energetically favored cis and trans isomers of prolyl bonds.
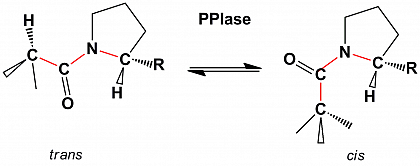
The cis/trans isomerization of prolyl bonds (prolyl isomerization) often forms a slow kinetic step during the folding or refolding of proteins. PPIases are important for the acceleration of these slow steps in protein folding.
Furthermore PPIases can interact with specific substrate proteins in the native state. By this PPIases can control many fundamental properties of their target proteins, like bioactivity, localization or stability. Thus, PPIases can thus influence a multitude of physiological and pathophysiological processes.
PPIases were discovered by Gunter Fischer in Halle in 1984. (https://de.wikipedia.org/wiki/Gunter_S._Fischer )
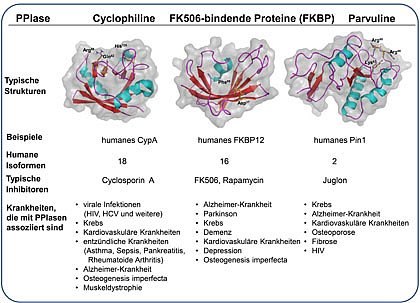
The enzyme class of PPIases (EC 5.2.1.8) include three different families, the cyclophilins, the FK506 binding proteins (FKBP) and the parvulins.
| Contact Cordelia Schiene-Fischer |
|---|

|
| phone: +49 345 55 22809 cordelia.schiene-fischer@biochemtech.uni-halle.de Department of Enzymology Germany |
| Postal address: Martin Luther University Halle-Wittenberg Institute for Biochemistry and Biotechnology Department of Enzymology Cordelia Schiene-Fischer 06099 Halle (Saale) Germany |





